News - Process Design
New publication - Setting commercialisation objectives for sustainable innovation
A new paper by Britest's Rob Peeling describes a methodology for setting commercialisation objectives for sustainability in an innovative project
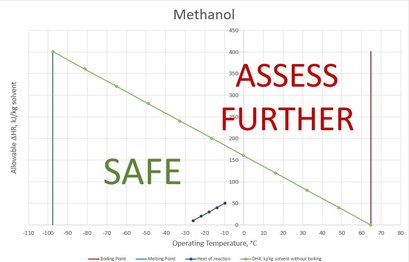
BatchSafe - a new tool for process safety screening
BatchSafe - a new tool for process safety screening Britest is delighted to announce the launch of a newly developed safety screening tool for solvent based batch reactions. Exclusively available to registered Britest Advocates, BatchSafe is an easy to use spreadsheet based utility that enables the
Britest and Micropore Technologies Announce Licence Agreement
Britest Limited has entered into a licensing agreement with Micropore Technologies. This allows Micropore to use a defined set of Britest’s established process understanding tools, in conjunction with their own proprietary technologies, to support their internal evaluation of potential applications of their membrane emulsification and encapsulation technology for client demonstration and general marketing purposes.
Ever felt the need to think more like a Chemical Engineer? This course could be for you...
Britest is providing an essential three-day training course for R&D Scientists and Engineers from other disciplines, who want to better understand the basic principles of chemical engineering, or make more effective considerations of whole process issues. Taking place 28th-30th April in Manchester.
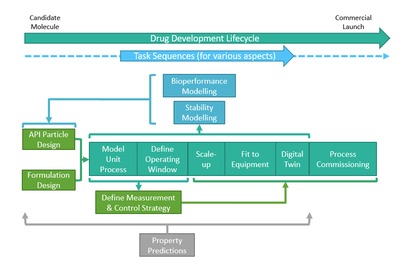
Article on digital information flows goes open access
Article on digital information flows goes open access A new publication describing how new digital models and approaches have been mapped onto the information workflow involved in pharmaceutical product and process design and development via a Britest-developed dedicated technical
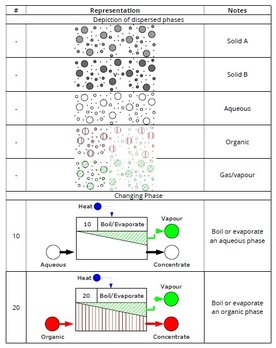
New task library expands armoury for visually presenting processes
A new "task library" supporting the use of the Britest Process Definition Diagram (PDD) tool substantially expands the armoury of ready-made task representations available to Britest facilitators and practitioners, and helps create better PDDs faster.
New online tool put continuous whole process design decision making in your hands
Newly released interactive tools for parallel decision making about whole process design and regulatory considerations around continuous manufacture are available now on the Britest website!
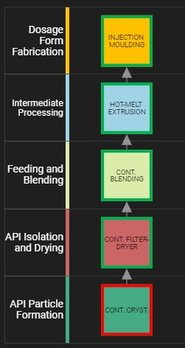
Focus article on digital design for pharmaceutical product and process development
Focus article on digital design for pharmaceutical product and process development An "in-depth focus" article in the June 2019 issue of European Pharmaceutical Review looks at how Britest has been working along with others in the ADDoPT collaborative consortium to explore how emerging process
Reflections on a continuous journey
As 2017 draws to a close, Britest's CEO Gareth Jenkins provides a timely reflection on the interwoven paths trodden towards widespread adoption of continuous manufacturing by the chemicals industry, and the development of the Britest toolkit for capturing and improving process understanding. Over the last few years, Britest has developed and applied a number of support tools for multiple-criterion based business decision making, and 2018 will see more case studies emerging,
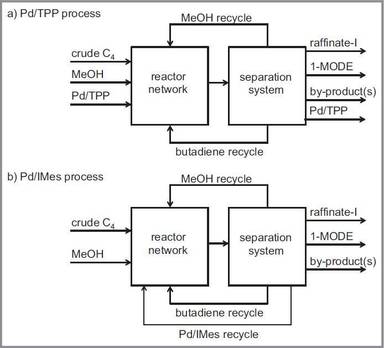
New conceptual study for butadiene telomerization published
Research arising from the EU's 7th Framework Programme’s SYNFLOW project on which Britest collaborated has resulted in a new, peer-reviewed publication describing a conceptual design study for a butadiene telomerization process.
Britest to frame thinking on new approaches to decision making for batch to continuous evaluation
Britest to frame thinking on new approaches to decision making for batch to continuous evaluation CEO speaking at 9th Symposium on Flow Chemistry for Industrial Applications Britest's Chief Executive Officer Dr. Gareth Jenkins will be in Barcelona next week, speaking at the latest in a series
Symposium Report - Continuous Flow Chemistry for Industrial Processes
Symposium Report - Continuous Flow Chemistry for Industrial Processes RSC Symposium, ChemSpec Europe, Münich, Germany, 31st May – 1st June 2017 The production of fine and speciality chemicals is still heavily reliant on batch processes. There is much technology innovation being developed