New online tool put continuous whole process design decision making in your hands
A newly released set of resources supporting the uptake of continuous manufacturing approaches in the pharmaceutical industry is available now as a free to use e-learning tool on the Britest website.
Developed by Britest and Cogent Skills in consultation with the ReMediES* consortium representing a broad supply and knowledge base in the sector, the tool provides an easy to use, self-guided environment by which you can explore relevant information and guidance around Parallel Decision making in Drug Product Whole Process Design and Regulatory Implications of Continuous Processing.

The Decision Making module developed by Britest not only summarizes the drivers around continuous manufacture and the range of process options available to manufacturers from API Particle formation through to dosage form fabrication, but allows you to interactively and iteratively explore these options and their implications on which avenues for upstream and downstream combinations are opened or closed by them. In this way, decisions can be made from a whole process perspective rather than simply locally optimizing single unit operations or process stages.
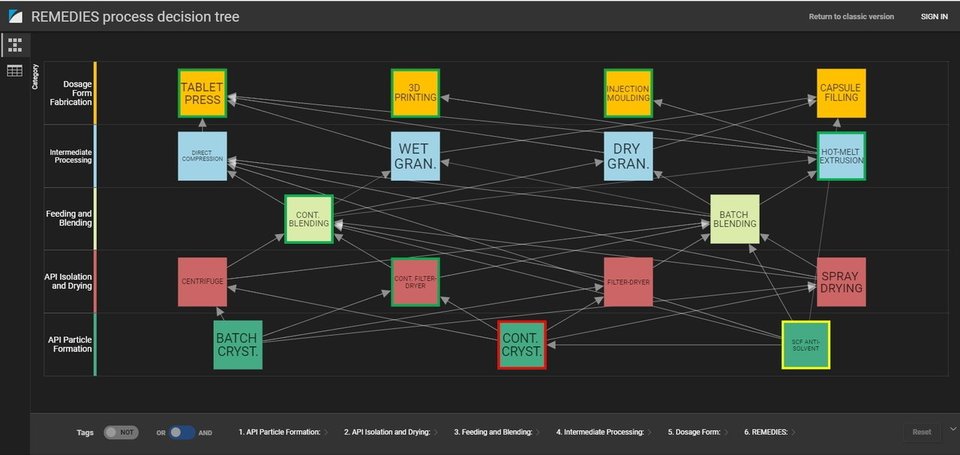
From a seemingly bewildering set of options......
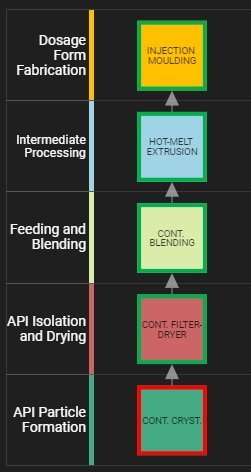
...a viable process conceptual design can be discerned.
The regulatory focused materials arose from work examining the skills implications of continuous manufacturing processes for skills and regulatory compliance. As part of this analysis, following discussions with the MHRA Cogent Skills identified a knowledge gap relating to regulatory expectations for continuous manufacturing. To address this gap, three sets of guidelines have been created: GMP Guidelines relating to Continuous Manufacturing, Quality by Design (QBD) and Development of a Continuous Manufacturing Process – Regulatory Considerations.
Go to ReMediES e-learning toolkit
