Continuous Flow Chiral Hydrogenation Scales Up Successfully
A new, high-profile scientific paper arising from one of Britest's international collaborative projects, which reports the first development of a continuous flow process for asymmetric hydrogenation with a heterogenized molecular catalyst in a real industrial context, has been selected as an “ACS Editors’ Choice” by the American Chemical Society. This recognition, based on recommendations by the scientific editors of ACS journals from around the world, means that the paper will remain open for all in the global community of researchers to access and read. You can access the full article here.
The publication in the ACS journal Organic Process Research & Development sees Britest Senior Innovation Specialist Charles Gordon co-author with others including the University of Nottingham’s Professor Sir Martyn Poliakoff FRS, Professor Walter Leitner and Dr. Giancarlo Franciò of RWTH Aachen University, and scientists from Pharmaceutical Development, AstraZeneca. It describes how continuous flow processing has been successfully used to achieve a key asymmetric step in the synthesis of an active pharmaceutical ingredient (API) of potential use in the treatment of cancer and inflammatory diseases robustly at kilogram scale, overcoming many of the limitations of a conventional batch process.
The authors report single-pass conversion rates in excess of 95.0% and enantioselectivity of more than 98.6% for an asymmetric hydrogenation reaction critical to the production of a previously clinically tested JAK2 kinase inhibitor. The synthesis was achieved using an electrostatically immobilized commercial catalyst (Rh/(S,S)-EthylDuphos).
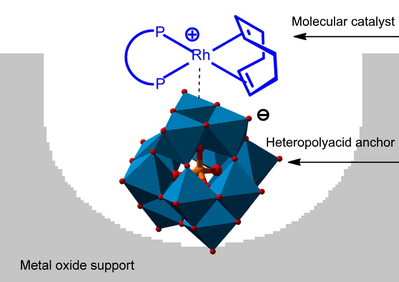 | |
A schematic depiction of the 'Augustine strategy' used to simplify and intensify the asymmetric hydrogenation. A molecular catalyst is anchored to a solid support (e.g., aluminum oxide) via a heteropolyacid linker (e.g. phosphotungstic acid). |
An automated, high-pressure, low-footprint pilot system was developed to carry out the asymmetric hydrogenation at kilogram-scale with a space time yield (STY) of up to 400 g l−1 h−1. Crucially, no catalyst leaching was detected in the product stream, thereby eliminating costly and time-consuming downstream purification procedures. This straightforward approach allowed for an easy and robust scale-up from gram to kilogram scale, fully matching the pharmaceutical quality criteria for enantiopurity and low metal content.
These exciting findings arise as a result of collaborative work within the EU's 7th Framework Programme’s SYNFLOW project. Overall, they provide a powerful demonstration of the massive potential and versatility of fully integrated continuous flow molecular catalysis; a “promising method” no longer, but rather a real world solution for process intensification in the chemical industry.