Technical facilitation provides information flow model for adoption of Digital Design approach in pharma
A recent presentation by Britest Senior Innovation Specialist Martin Edwards has outlined how a dedicated technical facilitation process designed and delivered by Britest has enabled a the development of an information flow for digital design and manufacture of pharmaceuticals. This is set to be a valuable enabler for the UK pharmaceutical industry in adopting new technology, and in building innovation and leadership capacity identified as essential to realise the potential of the Industry 4.0 revolution.
Presenting at the Formulation Science and Technology Group of the Royal Society of Chemistry's Formula X conference in Manchester, UK on the 25th June 2019, Dr. Edwards described how Britest had been working with partners from across the ADDoPT consortium to understand the needs of the industry in order to take up the benefits of digital design and operation and to formulate a suitable means of support in response.
Digital Design approaches offer a number of advantages in drug product design and manufacture. Essentially a “Digital Twin” of a product or process is created, and practical experiments (typically far fewer in number than conventionally) are used to parameterise and validate the model. Qualitative systems models can be used to help develop and test the assumptions of the quantitative model, and in the selection of parameters to explore in “virtual Design of Experiments".
Conducting virtual DoEs allows:
- Exploration of a wide range of material attributes and process parameters
- Identification of which parameters are critical to performance
- Assessment and optimisation of the robustness of performance with respect to variability in raw materials, physiology etc.
- Reduction in development time, costs and use of scarce materials
These are considerable attractions, but the digital route represents a significant culture shift for an industry where experimental development and validation pathways are long-embedded. The ADDoPT partners recognised the need for an overall framework that
- Allows users to understand the current state of models and their data requirements, and identifies potential future developments
- Combines the information flows from individual aspects of modelling and experimentation into an integrated structure
- Maps the digital design opportunities to the current product and process development workflows
- Links to training materials required to support the approach
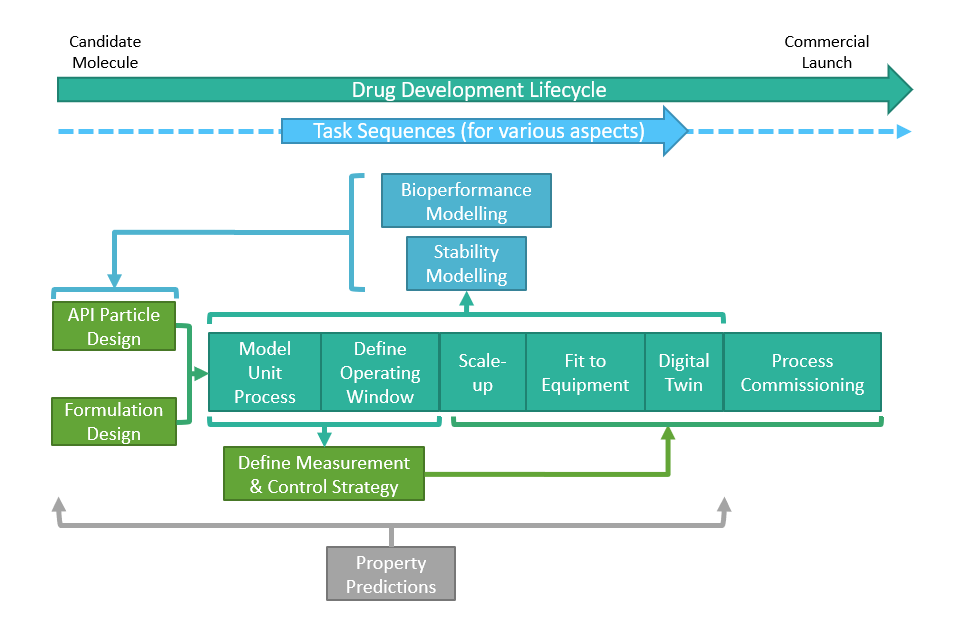
It is these needs that the new information flow addresses. Developed and implemented as an interactive, self-guided online flowchart, the information flow is extensively hyperlinked allowing the user to navigate flexibly and freely across tasks and to explore linkages between available models and tasks, via a visual relational database interface.
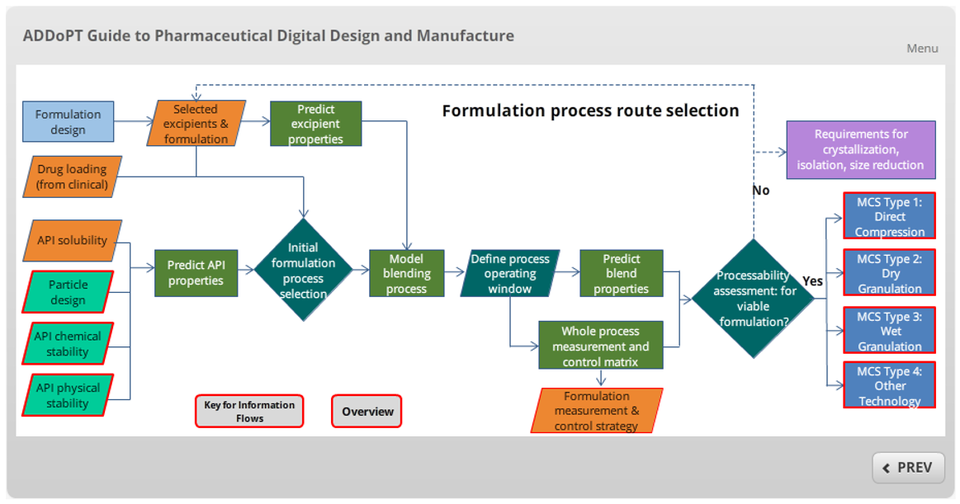
The Information Flow is flexibly navigable and highly user-interrogable
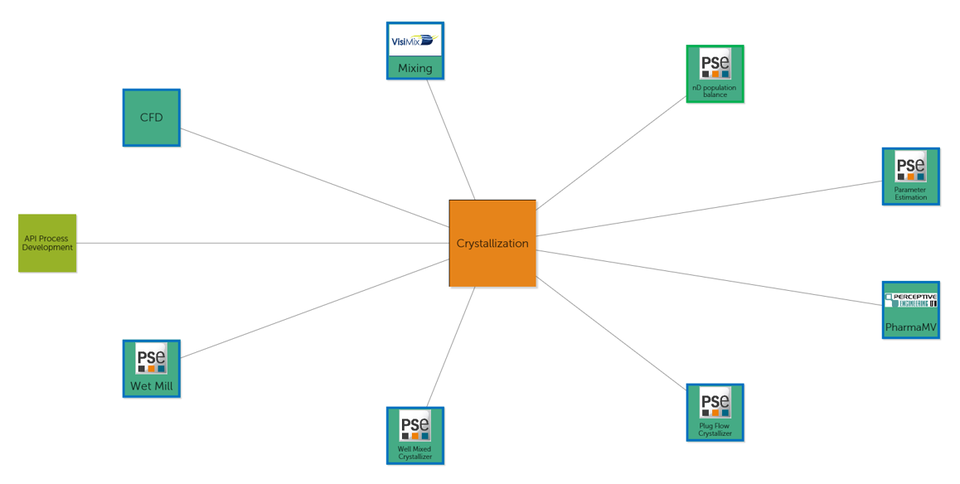
The generic ADDoPT Digital Information Flow will shortly be made available as a public legacy resource of the project. Within the project Britest has also worked directly with individual partners to develop information flows specific to their own processes and circumstances. Building on this experience, Britest believes that the approach taken could be readily extended to other sectors facing similar challenges in taking up the transformational advantages offered by digital approaches to product and process development and manufacture.
Martin Edward's presentation is available to view here.